Introduction to Bubble Continuous Positive
Airway Pressure (bCPAP) (Chapter 7/ E7)
See charts for CPAP
(TRY algorithm, increasing and weaning)
Learning Objectives
After completion of this chapter the participant should be able to:
Bubble Continuous Positive Airway Pressure (bCPAP)
Definition:
A process of giving continuous flow of air under regulated pressure through the airway.
- bCPAP stands for bubble continuous positive airway pressure.
- It is a constant pressure applied to the airway, generated by continuous, consistent
flow of air with the aim of opening collapsed lung segments and maintaining patency
in already opened air spaces.
E7 Bubble CPAP
Clinical Problem
Bubble CPAP (bCPAP) devices provide both positive pressure & increased
fractional concentration of oxygen (FiO2) to newborns with respiratory distress.
Bubble CPAP (bCPAP) is particularly useful for premature babies with
RDS: respiratory distress syndrome.(1.1)
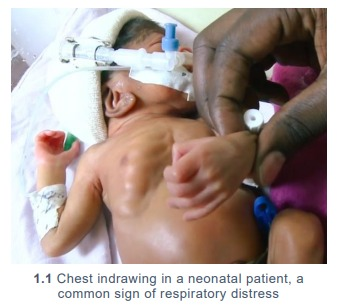
Very premature babies (<1.5kg and <32 weeks) benefit from early bCPAP. (Alert 1.1)
Reference can be made to the TRY algorithm. Depending on your facility
and your national policy the lowest weight at which to commence bCPAP may differ.
bCPAP should only be used when essential newborn care is in place, the equipment
is functioning, oxygen is available, staff are adequately trained in bCPAP, and close
monitoring can be assured.
| ? |
ALERT 1.1: When do you inititiate bCPAP vs low flow oxygen?
|
| Regarding the initiation of bCPAP versus low flow oxygen on the day of
birth, protocol developers should consider the following functions and
evidence. bCPAP functions to treat respiratory distress by improving
both ventilation (controlled provision of continuous inspiratory pressure)
and oxygenation [controlled provision of increased percentage of
oxygen - termed the fraction of inspired oxygen (FiO2)]. The positive
pressure from bCPAP prevents lung tissue (alveolae) from collapsing
on expiration thus improving ventilation, reducing the work of breathing
and preventing potentially irreversible lung damage.1 Additionally,
bCPAP has been shown to promote production of endogenous
surfactant,2 improve apnoea of prematurity3,4 and dramatically decrease
progression to mechanical ventilation,5-9 or death, in both high
income10,11 and low income 12-15 settings.
Thus, early bCPAP for preterm & small newborns, especially in settings
where mechanical ventilation and surfactant are unavailable, is critical
to prevent death. At a minimum, evidence points to preferential early
initiation of bCPAP, rather than low flow oxygen, in preterm newborns
under 1.5kg with any respiratory distress on the day of birth.
|
Bubble CPAP may also be used to treat neonatal patients with increased work of
breathing, designated by nasal flaring, grunting, head nodding, severe recession, RR
> 60, or an oxygen requirement of 0.5 to 1 L/min with peripheral blood saturations of
< 90%, in premature or term infants. (Alert 1.2)
Increased work of breathing may be caused by:
- Neonatal pneumonia
- Severe transient tachypnoea of the newborn
- Persistent pulmonary hypertension of the newborn
- Apnoea
of prematurity3,4
- Meconium aspiration syndrome
- Neonatal sepsis with severe respiratory distress
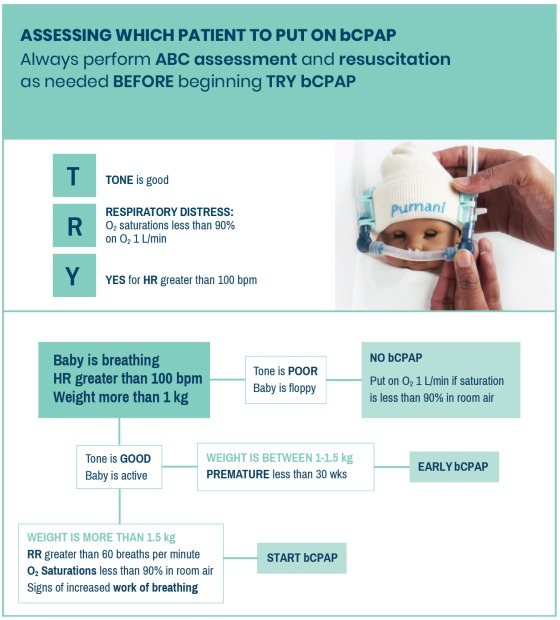
The TRY algorithm may be used by a nurse or clinician to decide who would benefit
from bCPAP and, if necessary, whom to prioritise. The TRY algorithm signs and
symptoms on which to act are straight forward and easily carried out. Premature
babies benefit most from CPAP and are given priority. Babies with severe asphyxia
leading to poor tone will not benefit. If CPAP machines are few in number, it is
important to provide CPAP to those who will benefit most. There are always exceptions
and in tertiary care units a paediatrician may decide to give CPAP to other infants.
Deciding when to start CPAP for a premature baby may differ between national
guidelines.
Contraindications to bCPAP include:
- Significant bleeding from mouth and nose
- Anatomical abnormalities of mouth and nose e.g., choanal atresia, cleft lip and palate
- Other obvious malformations incompatible with life
- Presence of a pneumothorax
Severely asphyxiated babies (with severe hypoxic ischaemic encephalopathy) do not
benefit from bCPAP.
| ? |
ALERT 1.2 bCPAP & low flow oxygen context
|
| Scale & delivery of neonatal care is critical. However, epidemiological
data has shown that rapid scale up of neonatal care without sufficient
attention to safety has long term negative consequences for neonatal
morbidity16 and is likely a contributor to the epidemic of preventable
blindness due to retinopathy of prematurity (ROP) in these settings.17
Supplemental oxygen is life-saving. However, when given in supraoptimal doses,
it has also been associated with ROP,18
bronchopulmonary dysplasia,19 periventricular leukomalacia and
prolonged ventilation.20 When using any form of oxygen therapy, it is
important to closely monitor blood oxygen saturation (SpO2) levels in
order to balance risks and benefits of supplemental oxygen. Exact blood
oxygen saturation targets for premature newborns remain an area of
controversy. However, most authorities agree that SpO 2 between 90-95%
is reasonable to minimise complications associated with low and high
oxygen levels.21-24
When choosing between low flow oxygen and bCPAP it is important to
keep the following physiological considerations in mind. Newborns under
2.5kg receiving low flow oxygen exceeding 0.5 L/min are administered
40-100% effective FiO2,25-28 which may increase morbidity. Delivery of
low flow oxygen in preterm newborns under 1.5kg has the added
complexity that positive pressure can be delivered even at flows as low
as 1-2.5 L/min.29,30 Unfortunately, as discussed above, at these rates of
low-flow oxygen, preterm newborns would be exposed to elevated levels
of effective FiO 2 which data show are likely to increase their morbidity.
In light of the above evidence and expert opinion, the recommendation
was made by our consortium to consider bCPAP in appropriate settings
when low flow oxygen greater than 0.5 - 1 L/min is required to maintain
saturations >90%. Of note, this recommendation is in line with the WHO
recommendation that a standard flow rate for neonates is 0.5 – 1L/min in
WHO Oxygen Therapy for Children;31 however, it is unaligned with the
suggestion to consider 4L/min of oxygen as the transition threshold from
nasal prongs to bCPAP.
bCPAP outside the neonatal period is not addressed by NEST360°
materials.
|
TRY BCPAP ALGORITHM
Zoom in to see details
Assessment
Respiratory distress can cause hypoxia contributing to both morbidity and
mortality. Bubble CPAP devices (2.1) use a pump to provide a blend of air and
oxygen at a continuous positive pressure. This pressure keeps airway spaces
open and increases alveolar recruitment throughout respiration in a
spontaneously breathing infant, which improves oxygenation and reduces work
of breathing.
Zoom in to see details

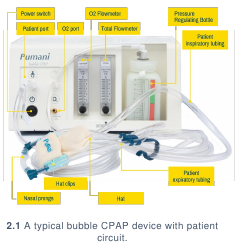
Traditional bCPAP devices are made up of the following components:
- Pump: pumps in air mixed with oxygen from an oxygen source.
- Inspiratory tube: connects the blended air and oxygen flow source to the patient.
- Expiratory tube: connects the patient to the pressure regulator.
- Pressure regulator: a water reservoir placed at the end of the expiratory circuit that
provides pressure using water level.
bCPAP devices range in complexity from vitals measured (e.g., saturations/respiratory
rates measured on the device) to outputs (e.g., humidified pressure vs pure pressure).
(Alert 2.1)
Pressures used in bCPAP devices range from 5 to 10 cm of water. As bCPAP delivers
a blend of air and oxygen, staff should also carefully monitor patients for oxygen
saturation using a pulse oximeter. Neonatal patients should reach oxygen saturations
of 90 – 95% by 15 minutes after birth. (Alert 2.2)
| ? |
ALERT 2.1 Use of humidification in bCPAP |
Some bCPAP units use heated and humidified gas in the circuit although
the exact benefits of humidification in non-invasive ventilation (i.e.,
bCPAP) in terms of survival, complications from therapy & morbidity are
not well established. For a more thorough review of theoretical
risk/benefits of heated humidified oxygen in bCPAP,
see Appendix 1.
Potential benefits of heating and humidification could include:
- Increased comfort and adherence.
- Decreased upper airway mucosal injury.
- Decreased convective heat losses which may lead to hypothermia
& challenging weight gain in infants.
- Decreased lung inflammation from mucus plugs which has
unknown impact on morbidity & mortality of very low birth weight
infants.
Potential drawbacks to heated humidification include:
- Hospital-acquired infection, especially in settings where clean
water may not be readily available and humidifiers, which are
typically meant for one-time use, are being cleaned and re-used
between patients.32
- High financial cost of adding heated humidified gas.33
- High human resource cost in terms of repair and preparation of
non-invasive ventilation units which may limit not only their use, but
availability of this life saving technology within our setting.33
In summary, based mostly on expert opinion, it is likely that heated and
humidified air is most important for the smallest newborns <1 -1.25kg
although this has never been explicitly studied. There is evidence from
Malawi that unheated un-humidified bCPAP can be used successfully to
decrease mortality of infants without excessive reports of upper airway
complications, but physiological implications in terms of morbidity and
mortality (hypothermia & weight gain) were not explicitly studied. Of note,
survival of infants >1.5kg on un-heated un-humidified air bCPAP in this
study12 were similar to survival of infants >1.5kg in Rwanda on heated
and humidified bCPAP.34
At this time, based on expert opinion and available literature, it does not
appear that the benefits of humidification outweigh the potential
risks/drawbacks for infants >1kg. Further study of the degree of humidity
provided by compressed air in various settings as well as implications of
humidification in low resource settings on iatrogenic infections, morbidity,
and mortality of neonates is needed.
|
| ? |
ALERT 2.2: SpO2 & Safe Oxygen Delivery |
When making this recommendation the following resources were
considered:
- According to the Textbook of Neonatal Resuscitation (NRP), 7th
Ed., “After birth, the oxygen saturation gradually increases above
90%. However, even healthy term newborns may take 10 minutes
or longer to reach this saturation” (p.77).35
- Target peripheral oxygen concentrations (SpO2) for newborns vary
depending on age and clinical condition. However, most authorities
agree that saturations between 90-95% minimises the
complications associated with both low and high oxygen levels
including death, neurodevelopmental impairment and Retinopathy
of Prematurity.21-24
|
Management
Management of bCPAP covers how to use the bCPAP device including set up
for a patient, patient preparation & commencement, care whilst on the device &
removal of the patient from the device.
SETTING UP FOR A PATIENT
- Collect: (3.1)
- bCPAP machine
- Power cable
- Inspiratory tubing
- Expiratory tubing
- CPAP prongs
- Connectors
- Oxygen tubing
- Oxygen source


- Position the bCPAP device at a secure location near the patient being
considered for bCPAP treatment. Plug the power cable into the back of the
machine (3.2) and plug into a socket or extension.



- Pull the bottle strap gently away from the bottle and remove the bottle. (3.3)
Unscrew the lid and fill with clean water to desired initial settings. (3.4) Most
patients will start with pressure levels of 6 cm of water. Re-screw the bottle lid
to the bottle and place back in bottle holder.
- Connect the inspiratory tubing to the Patient Port (indicated by the baby icon)
(3.5) and the expiratory tubing to the Bottle Port. (3.6)

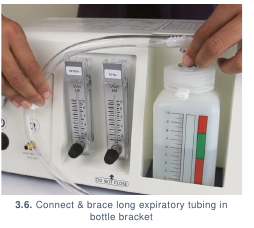
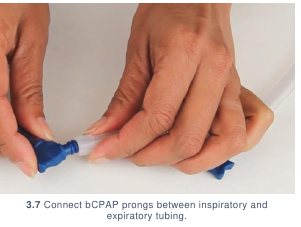
- Connect the CPAP prongs between the inspiratory and expiratory tubing. (3.7)
- Turn on the bCPAP device. (3.8)

- Open the oxygen flowmeter. Using oxygen tubing, connect the oxygen source
to the bCPAP device. (3.9)

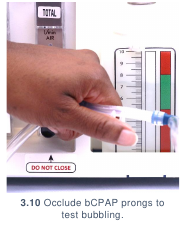
- Test the bubbling of the bCPAP device by occluding the CPAP prongs with your
fingers. (3.10) If the water within the water bottle bubbles, the bCPAP device is
ready for use.
PREPARING A PATIENT
- Place patient on oxygen and keep the baby warm whilst preparing for bCPAP.
- Always explain the purpose, risks, and benefits of a procedure to guardians BEFORE
performing the procedure.
- Follow handwashing protocol.
- Collect:
- Hat or length of stockinette (if hat is not available)
- Orogastric tube (OGT)
- Gloves
- Syringe
- Tape
- Suction catheter
- Correctly sized bCPAP prongs
- If a hat is not available, make a hat from a length of stockinette. (3.11)

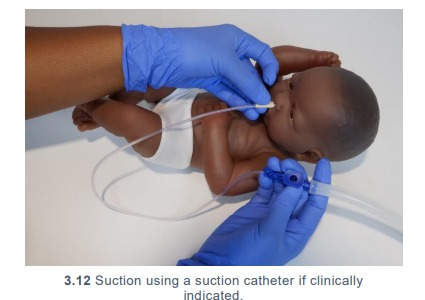
- Wash hands and put on gloves. Using suctioning guidelines, suction the
patient’s nose and mouth using the suction catheter if clinically indicated. (3.12)
- Insert an OGT: (Alert 3.1)
- Place the patient’s head in a neutral position, measure from the middle
of the mouth to the ear and then to halfway between the xiphisternum
and umbilicus. Mark this distance with a small amount of tape.
- Gently insert the OGT in the mouth to this length.
- Tape the OGT to the chin to keep in place. Use appropriate tape for
delicate newborn skin.
- Check placement of the OGT.36
- Using a syringe aspirate gastric contents. Test with litmus paper. The
litmus paper’s colour change should reflect an acidic pH (≤6); if it does
not, the OGT is incorrectly placed and should be re-sited. NOTE: Litmus
paper is manufactured in different colours. Acidic pH may be indicated by
different colour changes depending on manufacturer.
- If no gastric contents are aspirated, perform a whoosh test: push 2ml of air
down the OGT whilst listening over the abdomen with a stethoscope. If no
gurgling sound is heard the OGT is incorrectly placed and should be resited.
- If no gastric contents are aspirated and a whoosh test is not viable, an
alternative method is to place the OGT end into water. If continuous
bubbling occurs, the OGT is incorrectly placed and should be re-sited.
- Select bCPAP prong size from 000 to 5 based on nostril size. bCPAP prongs
should completely fill the patient’s nostrils. If prongs do not fill the nostril
completely, the pressure delivered to the patient will be decreased. If nostrils
turn a white colour the prongs are too tight and should be exchanged for the
next size down.
STARTING A PATIENT
- Collect: (3.13)
- Appropriately sized bCPAP prongs
- Hat
- 2-mL syringe filled with normal saline
- Hat clips OR
- 2 rubber bands & 4 safety pins
- Turn on the bCPAP device and connect oxygen source. Place hat on patient.
- Determine initial settings for the patient. Most patients will start with a pressure
level of 6 cm water, total flow of 6 L/min and oxygen concentration (FiO2) of
50%. Determine oxygen flow using FiO2 and total flow as shown on the oxygen
blending table printed on top of device. (3.14)

- Set total flow. Set oxygen flow on both oxygen source and bCPAP oxygen
flowmeter.
- Connect correctly sized bCPAP prongs to the inspiratory and expiratory tubing.
Retest the bubbling by pinching the bCPAP prongs shut.
- If the water within the pressure regulating bottle bubbles:
- Using syringe filled with saline, place a drop of saline within each nostril.
- Gently insert the prongs into the nostrils with the writing on the prongs facing
towards the caregiver.
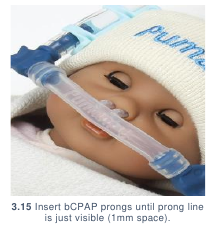
- bCPAP prongs should be inserted until the line on the bCPAP prongs is just
visible. This will leave 1 mm of space between the prongs and the nasal septum
to aid nasal patency. (3.15)
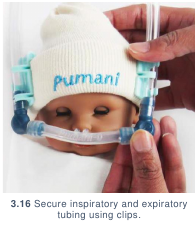
- Secure inspiratory and expiratory tubing to the patient using hat clips. (3.16) If
hat clips are unavailable, secure using rubber bands & safety pins:
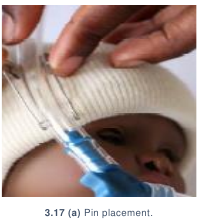
- Insert two safety pins on each side of the head in the brim of the hat. Pins
should open away from the baby’s face and should go only through the folded
brim of the hat. Pins should never touch the patient’s skin.
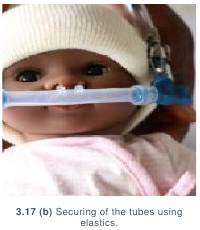
- Hold the inspiratory tubing in place between the two safety pins. Wrap the
rubber bands around the safety pins on either side of the tubing to secure.
Repeat for the expiratory tubing on the other side of the patient’s face. (3.17)
- Recheck that the prongs are still within the nose and inserted to the correct
distance from the nasal septum.
- Sometimes a small folded cloth placed under the baby’s shoulders prevents
the neck from bending and improves air/oxygen flow.
- Check that the water within the pressure regulating bottle bubbles. If it does not
bubble, check that the prongs completely fill the patient’s nostrils. If they do not,
replace with appropriately sized prongs.
- Monitor the patient 15 minutes after initiating bCPAP treatment and then 4
hourly for:
- Vital signs, including respiratory rate, heart rate, oxygen saturation and
temperature
- Work of breathing
- Nasal blockages
- Abdominal distension
- Act in accordance to clinical findings.
CARING FOR A PATIENT
Monitoring the patient should be completed 4 hourly, but may be required more
frequently depending on clinical condition. Monitoring should include:
- Vital signs, including respiratory rate, heart rate, oxygen saturation, and temperature
- Work of breathing
- Nasal blockages
- Abdominal distension
- Nasal septum trauma or breakdown
At every monitoring point:
- Provide a drop of saline to each nostril. (3.18)
- Check prongs, tubing & hat:
- Prongs should not be against nasal septum and check for skin compromise
- Tubing should not be kinked or misplaced
- Hat should not be loose; if it is loose, replace with new hat
- Check water level: if water level is below decided treatment level, add water
into bottle cap hole using a syringe or OG tube. (3.19) Water should be changed
daily.

Prior to increasing bCPAP always ensure the bCPAP device is functioning well and all
parts are in place. One mnemonic to help with this is DOPE:
- D: Displacement of prongs
- O: Obstruction of prongs or tubing
- P: Patient problem (e.g., pneumothorax)
- E: Equipment failure (e.g., power cut, tubing leak, see complications section)
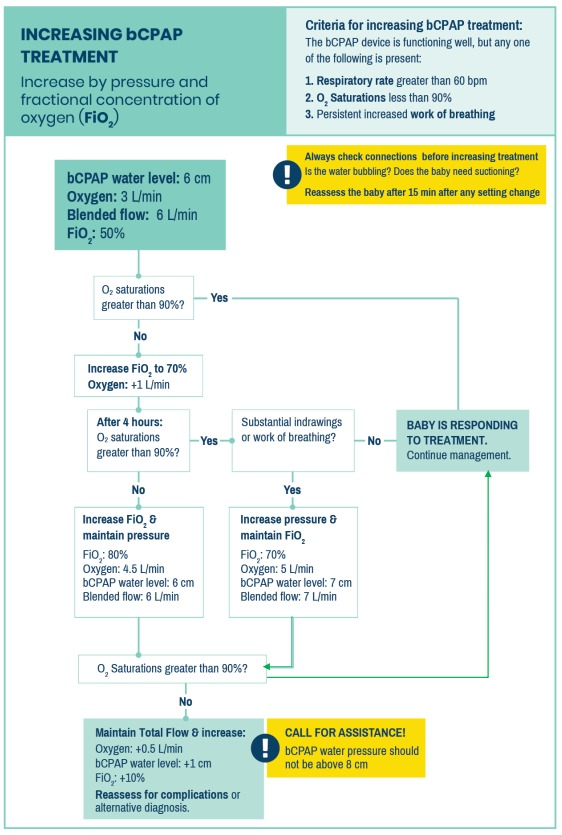
Increases in treatment are made in accordance to the Increasing bCPAP Treatment
algorithm.
INCREASING BCPAP
Zoom in for details.
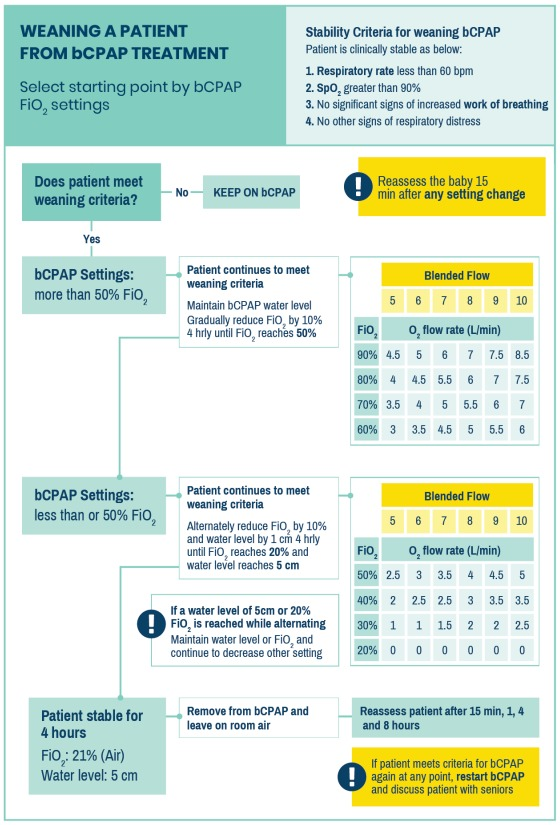
REMOVING A PATIENT
Refer to the following Weaning a Patient from bCPAP Treatment flow chart:
Zoom in for details.
Infection Prevention
Infection prevention, especially when using humidification or re-processing
respiratory circuits intended for single use, is CRITICAL to preventing
equipment related infections in newborns. If devices and equipment are not
disinfected or re-processed promptly or adequately between patients, they may
pose a significant infection risk.
GENERAL INFECTION PREVENTION
- Clean hands with soap and water or 70% alcohol before and after placing a
patient on bCPAP or handling any tubing that will be used on a patient.
- Ensure that all patient-related tubing (including prongs, inspiratory, and
expiratory tubing) is new or has been cleaned thoroughly and dried as per reuse
guidelines. (Alert 4.1) Any patient-related tubing must be cleaned before it
is used to place another patient on bCPAP. Nasal prongs are especially difficult
to clean thoroughly. Tubing should be hung to dry after disinfection and should
not touch the floor or other unsanitary surfaces whilst drying. Any item falling
on the floor is contaminated and must be cleaned thoroughly again.
- All patient-related consumables should be stored in a clean, dry location.
Tubing should be stored in loose rolls, preventing sharp bends or kinks which
will decrease the lifetime of the tubing.
| ? |
ALERT 4.1: Re-processing single-use devices
|
| Respiratory circuits and humidifiers associated with bCPAP are generally
intended as single use devices. However, in areas with limited resources
or challenging supply chains this equipment is often re-used. When reprocessing
single use devices it is extremely important that the process
is not delayed following completion of use. There should be a detailed
standard of practice as well as oversight processes for ensuring timely
and high-quality re-processing. If equipment is not re-processed promptly
or adequately between patients it poses a significant infection risk. Please
refer to the Reference Manual for Health Care Facilities with Limited
Resources Infection Prevention and Control Module 637 for more detailed
guidance on the re-processing of single use devices.
|
DISINFECTION AFTER USE
- Turn off bCPAP and dispose of water within pressure regulating water bottle.
- Dispose of hat and follow protocols for cleaning tubing if reusing prongs,
inspiratory and expiratory tubing. If patient consumables are not cleaned
thoroughly before using, infection can be transmitted. Care should be taken
particularly for consumables that are marked as single-use but are practically
reused.
- Clean the outside of the bCPAP device using a swab soaked in alcohol or
diluted chlorine. Total and oxygen flowmeter regulator controls should be
disinfected after each use using a cotton swab or gauze soaked in 70% alcohol.
Complications
Introduction of equipment in newborn care units poses clinical and device
complications for patients. Awareness of potential complications is critical to
maximise patient safety.
CLINICAL COMPLICATIONS
- Nasal blockage: the bCPAP prongs and nostrils can become blocked with mucus
which may result in increased respiratory distress and impaired oxygen delivery
resulting in hypoxia.
- Necrotic nasal septum: incorrectly sized or applied bCPAP prongs may result in
pressure on the nasal septum with resultant necrosis (tissue breakdown).
- Gastric distension: delivery of continuous airway pressure can cause gastric
distension and potential feed intolerance. The OGT should be closed for 30-60 min
after feeding but otherwise the OGT should be kept open on free drainage as this may
relieve distension.
- Pneumothorax: delivery of bCPAP occasionally causes a pneumothorax. If a patient
suddenly deteriorates whilst on bCPAP with increased respiratory distress and
worsening hypoxia assess for a pneumothorax.
- Decreased cardiac output: with excessive bCPAP levels, venous return may be
reduced resulting in decreased cardiac output.
DEVICE COMPLICATIONS
- Pressure leakages: if the water in the bottle is not bubbling, it is likely that the patient
is not getting therapeutic pressures. This may be due to the patient’s mouth being open
or bCPAP prongs not fully fitting the patient’s nostrils. It could also be caused by
kinking of the tube or a loose tube connection.
Bubble CPAP: Troubleshooting &
Repair| If the water in the bottle is not bubbling to identify and manage potential
causes for no bubbling.
- Power failure: bCPAP should ideally always be utilising outlets that have a source of
back-up power. If the power supply fails and patients are NOT on outlets with back-up
power they should be moved to outlets where back up power is available. If no back
up power is available the baby should receive oxygen from an oxygen cylinder until
they can be safely returned to bCPAP.
Care & Maintenance
Users are responsible for basic first-line care and maintenance to ensure
equipment lasts to their potential lifetime.
POWER SOURCE
Mains power.
WARD LOCATION
The bubble CPAP device should be secured in an easily accessible and visible
location near an oxygen source where nursing staff can regulate flows and manage
patients easily, but where it is not at risk of falling. All consumables required to place
a patient on bCPAP should be near the device and readily available to start treatment.
bCPAP devices vibrate during use; ensure that the vibration is not causing excess
sound (e.g., if placed on a table with metal instruments that will vibrate with the bCPAP
device).
USER PREVENTIVE MAINTENANCE
Minimal preventive maintenance is required for bCPAP devices. The bCPAP device
should be turned on weekly to a total flow of 10 L/min and allowed to run while
connected to an oxygen source at 2 L/min for at least 15 minutes. This is important to
ensure device functioning and minimise infection risk within internal respiratory
circuits.
Troubleshooting & Repair
Although users are not responsible for repairing their devices, there are steps
that may be taken to troubleshoot first-line errors that may occur before
contacting maintenance or engineering support.
| 1 |
The device does not turn on |
|
- Check that the power cable is securely attached (7.1) and connected to the
socket.
- Check that the power at the socket is turned on.
- If the machine still does not turn on, contact your maintenance team.
|

| 2 |
If the silver balls in the oxygen or total flowmeters are not going
up |
|
- Tap the front of the flowmeter firmly with your knuckle or the handle of a
screwdriver (or similar).
- If the flowmeter silver balls still do not go up, contact your maintenance
department to request cleaning of the flowmeter and to check that all internal
tubing is still connected.
|
| 3 |
If the total flowmeter does not go up to 10L/min |
|
- Contact your maintenance department to request an internal filter change.
|
| 4 |
If the water in the bottle is not bubbling |
|
- Check that the bCPAP prongs fully fill the nostrils and that the patient’s
mouth is not open. If the prongs do not fully fill the nostrils, replace the
prongs with a larger size.
- If the prongs are well-fitted, remove from the patient’s nose and occlude the
prongs with your finger. If the water is still not bubbling check the seal at the
patient port. If the seal is deteriorating or cracked (7.2), contact your
maintenance department to replace or troubleshoot further.
|

Appendix 1
Heated & Humidified Air in Non-Invasive Ventilation
When breathing, air is physiologically heated and humidified as it passes through our
upper airways into the lungs. Artificial heating and humidification are essential in
invasive ventilation (i.e., when using ventilators) which bypasses the upper airways.
However, the risks and benefits of heating and humidifying air supplied through non-invasive ventilator techniques such as Highflow Nasal Cannula (HFNC) and bCPAP
are not well established and currently there is not consensus about whether or not it
is a necessary element of all non-invasive ventilation systems.38,39
Benefits of heated and humidified gas in non-invasive ventilation may include
increased adherence and comfort.38 In neonates specifically, there is a physiological
argument that removal of heated and humidified gas may lead to increased convective
heat losses and therefore increased metabolic demand as well as increased insensible
fluid losses. One needs to consider if these effects may or may not have significant
effects on the infant’s ability to maintain their temperature and grow adequately during
the first weeks of life impacting mortality. In the long term, the effect of removing
humidification on morbidity of infants (specifically in terms of development of
bronchopulmonary dysplasia) is also unknown.40 Lastly, heated humidified bCPAP
may lead to lower incidence of mucosal injury which, in one study, was linked to
increased rates of sepsis in extremely low birthweight infants41; however, early data
from Malawi demonstrated few mucosal injuries when using un-heated un-humidified
bCPAP.12
Risks of humidification include a theoretical risk of infection especially in settings
where clean water may not be readily available and humidifiers, which are typically
meant for one time use, are being cleaned and re-used between patients.32 In addition,
humidification incurs a high financial cost as well as human resource costs in terms of
repair and preparation of non-invasive ventilation units which may limit not only their
use, but availability of this life saving technology within low resource settings.33
There is reason to believe that when supplying ambient air through the upper airway
there is in fact, no need for heated humidification.
33 This may be doubly true in low
resource bCPAP units such as the Pumani which, rather than using compressed air
sources (i.e., cylinders) are in fact driving flow through the circuit using compressed
ambient air.42 It is worth noting that although some lower cost bCPAP models do offer
passive humidification, expert opinion and experience suggests that perhaps the level
of humidification achieved via this method is not significant (data unpublished). Of
note, although the studies differ significantly, reported survival rates for infants >1.5kg
in Rwanda on a heated and humidified bCPAP circuit34 were similar to those reported
in Malawi on an un-heated, un-humidified circuit.12
In conclusion, despite recent WHO recommendations that bCPAP units should contain
humidification.31 In light of primary data which shows (1) the unknown necessity, (2)
the risks and benefits of heated and humidified gas in non-invasive ventilation, and (3)
the life-saving implications bCPAP has for neonates, our consortium maintains there
is a lack of evidence to resolve the question of humidification at this time. Further study
of the degree of humidity provided by compressed air in various settings as well as
implications of humidification in low resource settings on iatrogenic infections,
morbidity and mortality of neonates is needed. It is important that when considering
implementation of bCPAP, one considers not only physiological implications of this
feature in the bCPAP units, but also how this feature impacts supply chain, human
resource costs, financial costs, training, infection control, maintenance, and availability
of units in country.
References
- Subramaniam, P., Ho, J. J. & Davis, P. G. Prophylactic nasal continuous positive airway
pressure for preventing morbidity and mortality in very preterm infants. Cochrane Database of
Systematic Reviews (2016) doi:10.1002/14651858.CD001243.pub3.
- Mulrooney, N. et al. Surfactant and Physiologic Responses of Preterm Lambs to Continuous
Positive Airway Pressure. American Journal of Respiratory and Critical Care Medicine 171,
488–493 (2005).
- Miller, M. J., Carlo, W. A. & Martin, R. J. Continuous positive airway pressure selectively
reduces obstructive apnea in preterm infants. J. Pediatr. 106, 91–94 (1985).
- Kattwinkel, J., Nearman, H. S., Fanaroff, A. A., Katona, P. G. & Klaus, M. H. Apnea of
prematurity. Comparative therapeutic effects of cutaneous stimulation and nasal continuous
positive airway pressure. J. Pediatr. 86, 588–592 (1975).
- Gittermann, M. K., Fusch, C., Gittermann, A. R., Regazzoni, B. M. & Moessinger, A. C. Early
nasal continuous positive airway pressure treatment reduces the need for intubation in very low
birth weight infants. Eur. J. Pediatr. 156, 384–388 (1997).
- Jónsson, B., Katz-Salamon, M., Faxelius, G., Broberger, U. & Lagercrantz, H. Neonatal care of
very-low-birthweight infants in special-care units and neonatal intensive-care units in
Stockholm. Early nasal continuous positive airway pressure versus mechanical ventilation:
gains and losses. Acta Paediatr Suppl 419, 4–10 (1997).
- te Pas, A. B., Spaans, V. M., Rijken, M., Morley, C. J. & Walther, F. J. Early nasal continuous
positive airway pressure and low threshold for intubation in very preterm infants. Acta Paediatr.
97, 1049–1054 (2008).
- Narendran, V. et al. Early bubble CPAP and outcomes in ELBW preterm infants. J Perinatol
23, 195–199 (2003).
- Hascoet, J.-M., Espagne, S. & Hamon, I. CPAP and the preterm infant: Lessons from the COIN
trial and other studies. Early Human Development 84, 791–793 (2008).
- Ho, J. J., Subramaniam, P. & Davis, P. G. Continuous distending pressure for respiratory
distress in preterm infants. Cochrane Database of Systematic Reviews (2015)
doi:10.1002/14651858.CD002271.pub2.
- Sekar, K. C. & Corff, K. E. To tube or not to tube babies with respiratory distress syndrome.
Journal of Perinatology 29, S68–S72 (2009).
- Kawaza, K. et al. Efficacy of a Low-Cost Bubble CPAP System in Treatment of Respiratory
Distress in a Neonatal Ward in Malawi. PLoS ONE 9, e86327 (2014).
- Koyamaibole, L. An Evaluation of Bubble-CPAP in a Neonatal Unit in a Developing Country:
Effective Respiratory Support That Can Be Applied By Nurses. Journal of Tropical Pediatrics
52, 249–253 (2005).
- Martin, S., Duke, T. & Davis, P. Efficacy and safety of bubble CPAP in neonatal care in low and
middle income countries: a systematic review. Archives of Disease in Childhood - Fetal and
Neonatal Edition 99, F495–F504 (2014).
- Okello, F. et al. Reducing preterm mortality in eastern Uganda: the impact of introducing low-cost bubble CPAP on neonates <1500 g. BMC Pediatrics 19, (2019).
- Lawn, J. E., Blencowe, H., Darmstadt, G. L. & Bhutta, Z. A. Beyond newborn survival: the world
you are born into determines your risk of disability-free survival. Pediatric Research 74, 1–3
(2013).
- Gilbert, C. et al. Epidemiology of ROP update – Africa is the new frontier. Seminars in
Perinatology (2019) doi:10.1053/j.semperi.2019.05.002.
- Flynn, J. T. et al. A cohort study of transcutaneous oxygen tension and the incidence and
severity of retinopathy of prematurity. N. Engl. J. Med. 326, 1050–1054 (1992).
- SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network et
al. Target ranges of oxygen saturation in extremely preterm infants. N. Engl. J. Med. 362, 1959–
1969 (2010).
- Kayton, A., Timoney, P., Vargo, L. & Perez, J. A. A Review of Oxygen Physiology and
Appropriate Management of Oxygen Levels in Premature Neonates: Advances in Neonatal
Care 18, 98–104 (2018).
- Cummings, J. J., Polin, R. A. & Committee on Fetus and Newborn. Oxygen Targeting in
Extremely Low Birth Weight Infants. Pediatrics 138, e20161576 (2016).
- Bancalari, E. & Claure, N. Oxygenation Targets and Outcomes in Premature Infants. JAMA
309, 2161 (2013).
- Manja, V., Lakshminrusimha, S. & Cook, D. J. Oxygen Saturation Target Range for Extremely
Preterm Infants: A Systematic Review and Meta-analysis. JAMA Pediatrics 169, 332 (2015).
- Polin, R. A. & Bateman, D. Oxygen-Saturation Targets in Preterm Infants. New England Journal
of Medicine 368, 2141–2142 (2013).
- Fan, L. L. & Voyles, J. B. Determination of inspired oxygen delivered by nasal cannula in infants
with chronic lung disease. The Journal of Pediatrics 103, 923–925 (1983).
- Benaron, D. A. Maximizing the Stability of Oxygen Delivered Via Nasal Cannula. Archives of
Pediatrics & Adolescent Medicine 148, 294 (1994).
- The STOP-ROP Multicenter Study Group. Supplemental Therapeutic Oxygen for Prethreshold
Retinopathy of Prematurity (STOP-ROP), A Randomized, Controlled Trial. I: Primary
Outcomes. Pediatrics 105, 295–310 (2000).
- Walsh, M. Oxygen Delivery Through Nasal Cannulae to Preterm Infants: Can Practice Be
Improved? Pediatrics 116, 857–861 (2005).
- Locke, R. G., Wolfson, M. R., Shaffer, T. H., Rubenstein, S. D. & Greenspan, J. S. Inadvertent
administration of positive end-distending pressure during nasal cannula flow. Pediatrics 91,
135–138 (1993).
- Sreenan, C., Lemke, R. P., Hudson-Mason, A. & Osiovich, H. High-Flow Nasal Cannulae in the
Management of Apnea of Prematurity: A Comparison With Conventional Nasal Continuous
Positive Airway Pressure. Pediatrics 107, 1081–1083 (2001).
- Oxygen therapy for children. (World Health Organization, 2016).
- Dreimanis, D., Beckingham, W., Collignon, P. & Graham, M. Review of neonatal unit continuous
positive airways pressure (CPAP)(with humidification). Healthcare Infection 11, 22–23 (2006).
- Branson, R. D. & Gentile, M. A. Is humidification always necessary during noninvasive
ventilation in the hospital? Respiratory Care 55, 209–216 (2010).
- Nahimana, E. et al. Bubble CPAP to support preterm infants in rural Rwanda: a retrospective
cohort study. BMC Pediatrics 15, (2015).
- Textbook of Neonatal Resuscitation (NRP), 7th Ed. (American Academy of Pediatrics, 2016).
- Pocket book of hospital care for children: guidelines for the management of common childhood
illnesses. (World Health Organization, 2013).
- Curless MS, Ruparelia CS, Thompson E, and Trexler PA, eds. 2018. Infection Prevention and
Control: Reference Manual for Health Care Facilities with Limited Resources. Jhpiego:
Baltimore, MD.
- Restrepo, R. D. & Walsh, B. K. Humidification during invasive and noninvasive mechanical
ventilation: 2012. Respiratory Care 57, 782–788 (2012).
- Roberts, C. T. et al. The effects of non-invasive respiratory support on oropharyngeal
temperature and humidity: a neonatal manikin study. Archives of Disease in Childhood - Fetal
and Neonatal Edition 101, F248–F252 (2016).
- Shaffer, T. H., Alapati, D., Greenspan, J. S. & Wolfson, M. R. Neonatal non-invasive respiratory
support: Physiological implications. Pediatric Pulmonology 47,
837–847 (2012).
- Kopelman, A. E. & Holbert, D. Use of Oxygen Cannulas in Extremely Low Birthweight Infants
is Associated with Mucosal Trauma and Bleeding, and Possibly with Coagulase-negative
Staphylococcal Sepsis. Journal of Perinatology 23, 94–97 (2003).
- Brown, J. et al. A High-Value, Low-Cost Bubble Continuous Positive Airway Pressure System
for Low-Resource Settings: Technical Assessment and Initial Case Reports. PLoS ONE 8,
e53622 (2013).




























